Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:

Snakebite antivenom was first produced in South Africa in 1901 and in small quantities. For the next 30-odd years, antivenom was largely imported from the Pasteur Institute in France. During that period, a 10 ml vial of cobra or mamba antivenom could be purchased from Mr. F.W. Fitzsimons, Director of the Port Elizabeth Museum.
In 1928, the South African Institute of Medical Research (SAIMR) started antivenom production. They experimented with a variety of domestic animals for serum production, but ultimately settled on horses, largely because of the large volume of blood that can be tapped during a session. Horses are made hyperimmune to snake venom by injecting them with small quantities of venom at a time, gradually increasing the dosages as the horse builds up resistance to the venom. Once the horse is immune, a process that takes about nine months, blood is taken from it and the serum removed from the blood cells and purified.

There were various antivenoms produced over the years, but in 1971, SAIMR produced a polyvalent antivenom which, in its preparation, used venom from the Snouted Cobra, Forest Cobra, Mozambique Spitting Cobra and the Black-necked Spitting Cobra. This was changed at a later stage, and the venom from the Green Mamba, Black Mamba, Jameson’s Mamba, Cape Cobra, Snouted Cobra, Forest Cobra, Mozambique Spitting Cobra, Rinkhals, Puff Adder and Gaboon Adder was used in the production of the current polyvalent antivenom. A monovalent antivenom, which is effective against the venom of the Boomslang, was produced in 1940
The SAIMR was rebranded as the National Health Laboratories, and venom production was done by the South African Vaccine Producers, a government department.
In cases of serious snakebite envenomation, it is recommended that victims bitten by highly venomous snakes with cytotoxic venom receive 6 vials of SAVP polyvalent antivenom, and those bitten by a snake with neurotoxic venom should initially be treated with 12 vials. More antivenom may be required at a later stage.
Boomslang victims should ideally be treated with two vials of monovalent Boomslang antivenom. The antivenom is given intravenously via a saline drip and usually over half an hour.

Both SAVP antivenoms are supplied in liquid form in a 10 ml glass vial and must always be refrigerated (not frozen) below 10 degrees centigrade. The shelf life of these antivenoms is three years, after which it may not be legally used on humans. Provided the expired antivenom remains clear and not cloudy or milky, it can be used by vets on pets with success. Most snakebite victims who are hospitalised after a snakebite are not treated with antivenom, as not all bites are severe. Doctors will closely monitor the patient’s symptoms and administer antivenom if required. Up to 40% of snakebite victims that are treated with SAVP antivenom have a severe allergic reaction, and for this reason, antivenom is only administered by a medical professional in a hospital environment.
There have been many instances in the past when the SAVP ran short of antivenom, and orders were often delayed for a few weeks. However, since COVID, they have experienced severe production problems, and for the past year or so, SAVP have not produced any antivenom. They no longer have stock of SAVP polyvalent antivenom and, in their last correspondence, mentioned that they are busy upgrading their facilities and hope to be back in production by April 2025. This never happened, and at this stage, it is doubtful that they will ever produce antivenom again.

There are several antivenom producers in India and South America that produce antivenoms which are sold in Africa. Most of these antivenoms perform rather poorly when it comes to neutralising the venom of southern African snakes, and none of them have been subjected to clinical trials.
For these products, which are not registered with the South African Health Products Regulatory Authority (SAPHRA), they must be imported under section 21 of SAHPRA, and this requires a great deal of paperwork. Only one product, PANAF Polyvalent, is currently in the process of being registered with SAPHRA in South Africa for human use.
PANAF Polyvalent has been produced since 2015 and is currently the only World Health Organisation (WHO) approved snake antivenom for use in Africa, and has an internationally recognised GMP code (Good Manufacturing Practice). It is sold in powder form with sterile water, can be stored at room temperature up to 30 degrees centigrade and has a shelf life of four years. It covers 24 African species, including the Puff Adder, Gaboon Adder, our cobras and mambas, and provides cross-neutralisation for the Cape Cobra and Rinkhals, although their venoms are not, as yet, added to the mix.
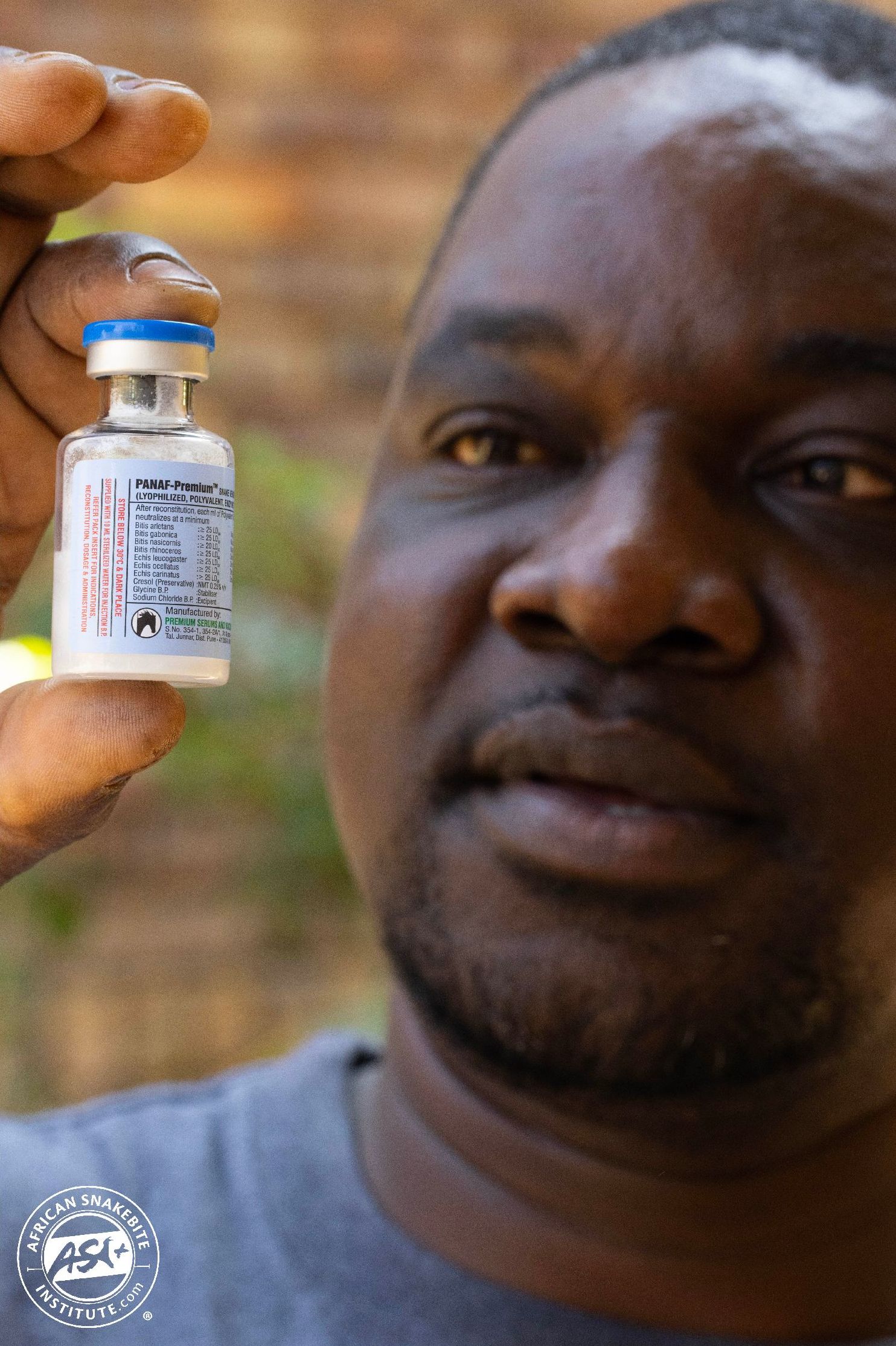
PANAF has already been registered in Namibia and Botswana and is in the process of being fully registered in South Africa with SAPHRA. This product is already being used in our private and state hospitals and by veterinarians.
The cost of PANAF Polyvalent antivenom is like that of SAVP polyvalent antivenom, around R2,000.00 per vial. The recommended initial dosages are somewhat higher – 10 vials for Puff Adder bites, 10-15 vials for Black and Green Mamba bites, 10 vials for Mozambique Spitting Cobra bites and 10-20 vials for Cape Cobra bites. One major plus side is that we have not recorded allergic reactions to this product so far. At this stage, PANAF does not produce antivenom for Boomslang bites.
PANAF is already in use in Zimbabwe, Angola, Mozambique, Zambia, Namibia, Botswana and various other sub-Saharan countries.
CONTACT US:
Product enquiries:
Caylen White
+27 60 957 2713
info@asiorg.co.za
Public Courses and Corporate training:
Michelle Pretorius
+27 64 704 7229
courses@asiorg.co.za
 ASI Lite Combo 3
R1,575.00
ASI Lite Combo 3
R1,575.00
 ASI Collapsible Snake Hook - 1.2 m
R650.00
ASI Collapsible Snake Hook - 1.2 m
R650.00
 Rangers Book Combo 1
Rangers Book Combo 1
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Want to attend the course but can’t make it on this date?
Fill in your details below and we’ll notify you when we next present a course in this area:
Sign up to have our free monthly newsletter delivered to your inbox:
Before you download this resource, please enter your details:
Before you download this resource, would you like to join our email newsletter list?